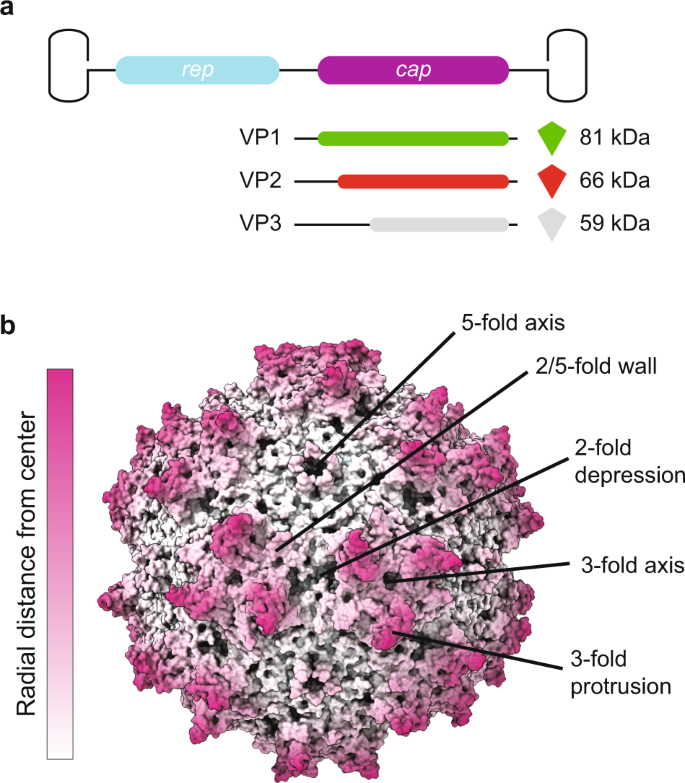
Engineering AAV vectors for enhanced safety profiles involves multiple strategies at both the vector genome and capsid levels. Here is a breakdown of these strategies:
Vector Genome Level:
- Modifying Vector Genome Sequences: Scientists modify AAV vector genomes by adding, mutating, or deleting specific sequences. For example, self-complementary AAV (scAAV) vectors are designed by deleting key signals from the second inverted terminal repeat (ITR), allowing for more efficient genome replication.
- Codon Optimization: Optimizing the codon usage of the transgene can enhance its expression efficiency.
- Promoter and PolyA Sequence Selection: Careful selection and manipulation of promoter and polyadenylation (polyA) sequences can influence transgene expression and tissue specificity.
Capsid Engineering:
Capsid engineering strategies can be categorized into four main categories:
- Directed Evolution: This approach involves creating capsid mutant libraries using error-prone PCR or introducing peptides with known or speculated affinities into the capsid. It relies on selecting capsids that exhibit desired properties.
- Rational Design: Scientists use knowledge of capsid structure and function to make precise modifications to improve capsid properties. This approach is guided by insights from structural studies.
- Computer-Guided Design: Computational modeling and simulations help predict how specific capsid modifications may affect interactions with host cells and the immune system.
- Combinations: Combining different strategies, such as directed evolution and rational design, can optimize capsids for specific purposes.
Circumventing Immune Responses:
AAV vectors, despite low immunogenicity, can still trigger immune responses that affect safety. Strategies to address these issues include:
- CpG Motif Modification: Unmethylated CpG motifs in AAV vectors can activate immune responses. Modifying the vector's genome to reduce CpG motifs can mitigate this risk.
- Innate Immunity Sensors: Understanding how AAV vectors interact with innate immunity sensors like TLR9 and MDA5 can help design vectors that minimize immune activation.
- Neutralizing Antibody Escape: Some strategies focus on escaping neutralizing antibodies (NAbs) to maintain vector efficacy. Directed evolution and rational design can generate AAV variants that are less susceptible to NAbs.
- Complement Activation: Complement activation can pose risks. Capsid engineering approaches aim to understand and modify AAV capsids to reduce complement activation, especially in the alternative pathway.
Minimizing Genotoxicity:
- Integration Control: While AAV vectors typically do not integrate into the host genome, there is a low risk of genotoxicity. Scientists explore methods to control integration, including using homology arms for targeted integration and CRISPR-Cas9 combinations.
- Partial Genomes: Strategies are devised to prevent the generation of partial genomes, which may lead to genotoxicity. Inverted genomes for self-complementary AAVs and deleting promoter-proximal D-sequences for single-stranded AAVs are considered.
- Promoter and Enhancer Selection: The choice of promoters and enhancers can affect the risk of genotoxicity. Some regulatory elements may lead to overexpression or oncogenicity, while others can be used to limit transgene expression to safe levels.
Controlling On-Target Delivery and Off-Target Expression:
- Tissue-Specific Promoters: Using tissue-specific promoters or regulatory elements ensures that gene expression is limited to the target tissue, reducing off-target effects.
- Clonal Selection: Clonal selection techniques help identify AAV clones that exhibit the highest transduction efficiency in the target tissue while minimizing off-target transduction.
- Balancing Safety and Efficacy: Striking the right balance between transduction efficiency and safety is crucial, especially when using high vector doses or systemic delivery.
In summary, engineering AAV vectors for improved safety profiles involves a multifaceted approach that encompasses vector genome modifications, capsid engineering, immune response mitigation, genotoxicity control, and precise control over on-target and off-target transduction. These strategies aim to enhance the safety and efficacy of AAV-based gene therapies.
For details
https://www.frontiersin.org/articles/10.3389/fmmed.2022.1054069/full